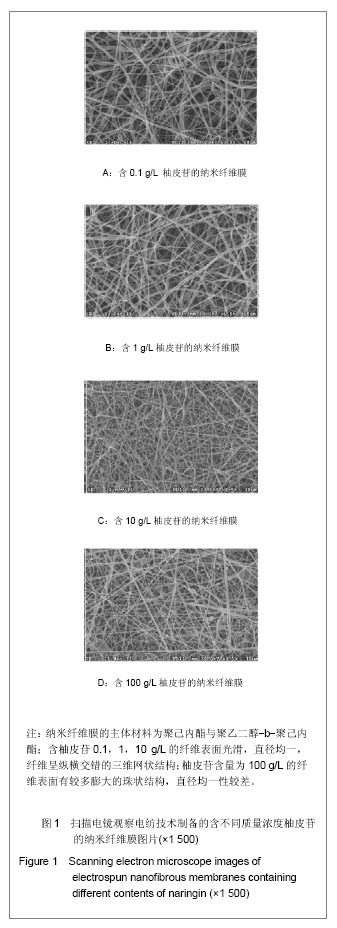
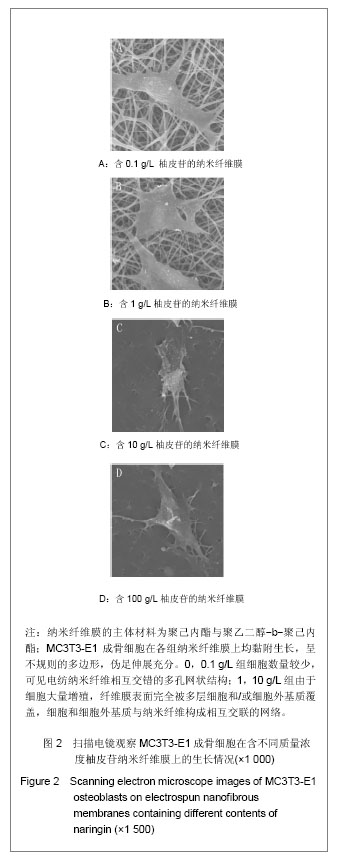
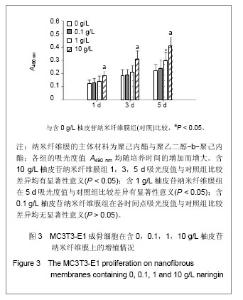
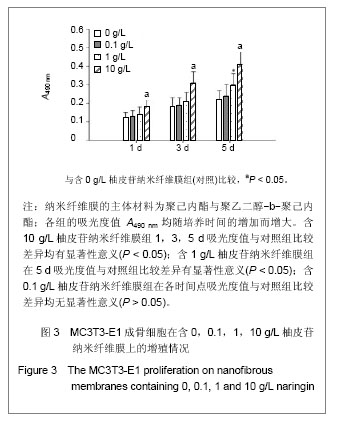
| [1] Erdogan O,Shafer DM,Taxel P,et al.A review of the association between osteoporosis and alveolar ridge augmentation.Oral Surg Oral Med Oral Pathol Oral Radiol Endod.2007;104(6): 738 e731-713.[2] Chang DY,Dong FH. Jilin Zhongyiyao.2006;26(6):61-62.常德有,董福慧.骨碎补的研究概况[J].吉林中医药,2006,26(6): 61-62.[3] Wang XL,Wang NL,Zhang Y,et al.Effects of eleven flavonoids from the osteoprotective fraction of Drynaria fortunei (KUNZE) J. SM. on osteoblastic proliferation using an osteoblast-like cell line. Chem Pharm Bull (Tokyo). 2008; 56(1): 46-51.[4] Jeong JC,Lee JW,Yoon CH,et al.Stimulative effects of Drynariae Rhizoma extracts on the proliferation and differentiation of osteoblastic MC3T3-E1 cells.J Ethnopharmacol. 2005; 96(3): 489-495.[5] Wu JB,Fong YC,Tsai HY,et al.Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol. 2008; 588(2-3): 333-341.[6] Hirata M. Naringin suppresses osteoclast formation and enhances bone mass in mice. J Health Sci. 2009; 55(3): 463-467.[7] Venugopal J,Low S,Choon AT,et al.Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J Biomed Mater Res A. 2008; 85(2): 408-417.[8] Sui G,Yang X,Mei F,et al. Poly-L-lactic acid/hydroxyapatite hybrid membrane for bone tissue regeneration.J Biomed Mater Res A. 2007; 82(2): 445-454.[9] Nie H,Soh BW,Fu YC,et al.Three-dimensional fibrous PLGA/HAp composite scaffold for BMP-2 delivery. Biotechnol Bioeng.2008;99(1): 223-234.[10] Sahoo S,Ouyang H,Goh JC,et al.Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng.2006;12(1): 91-99.[11] Lee SJ,Yoo JJ,Lim GJ,et al.In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application.J Biomed Mater Res A.2007;83(4): 999-1008.[12] Schnell E,Klinkhammer K,Balzer S,et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend.Biomaterials. 2007; 28(19): 3012-3025.[13] Christenson EM,Anseth KS,van den Beucken JJ,et al.Nanobiomaterial applications in orthopedics.J Orthop Res.2007; 25(1): 11-22.[14] Chen FM,Jin Y,Wu ZF. Yati Yasui Yazhou Bingxue Zazhi. 2004; 14(10):589-592.陈发明,金岩,吴织芬. 生长因子复合生物膜引导牙周组织再生[J].牙体牙髓牙周病学杂志,2004,14(10): 589-592.[15] Jovanovic SA,Hunt DR,Bernard GW,et al.Bone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defects. Clin Oral Implants Res.2007;18(2): 224-230.[16] Wong RW,Rabie AB.Effect of naringin on bone cells. J Orthop Res. 2006; 24(11): 2045-2050.[17] Wei M,Z Yang,P Li,et al. Anti-osteoporosis activity of naringin in the retinoic acid-induced osteoporosis model. Am J Chin Med. 2007; 35(4): 663-667.[18] Ang ES,Yang X,Chen H,et al.Naringin abrogates osteoclastogenesis and bone resorption via the inhibition of RANKL-induced NF-kappaB and ERK activation. FEBS Lett. 2011; 585(17): 2755-2762.[19] Guo D,Wang J,Wang X,et al.Double directional adjusting estrogenic effect of naringin from Rhizoma drynariae (Gusuibu). J Ethnopharmacol.2011; 138(2): 451-457.[20] Yoshimoto H,Shin YM,Terai H,et al.A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003; 24(12): 2077-2082.[21] Wang WS,Ren XJ.Huagong Jinzhan. 2004; 23(12): 1316-1319.王文生,任笑杰.双亲嵌段聚合物的合成及在共混体系中的增容作用[J].化工进展, 2004,23(12): 1316-1319.[22] Kim K,Luu YK,Chang C,et al.Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds.J Control Release. 2004; 98(1): 47-56.[23] Jeong SI,Kim SY,Cho SK,et al.Tissue-engineered vascular grafts composed of marine collagen and PLGA fibers using pulsatile perfusion bioreactors. Biomaterials.2007; 28(6): 1115-1122.[24] Zhao J,Jiang XQ,Zhang ZY.Zhongguo Kouqiang Hemian Waike Zazhi.2008; 6(2):137-140.赵君,蒋欣泉,张志愿. 纳米级支架材料在骨组织工程中的应用[J].中国口腔颌面外科杂志,2008, 6(2): 137-140.[25] Chen LL,Lei LH,Ding PH,et al.Osteogenic effect of Drynariae rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch Oral Biol.2011; 56(12): 1655-1662.[26] Zhang P,Dai KR,Yan SG,et al.Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009; 607(1-3): 1-5.[27] Luben RA,Wong GL,Cohn DV. Biochemical characterization with parathormone and calcitonin of isolated bone cells: provisional identification of osteoclasts and osteoblasts. Endocrinology. 1976; 99(2): 526-534. |